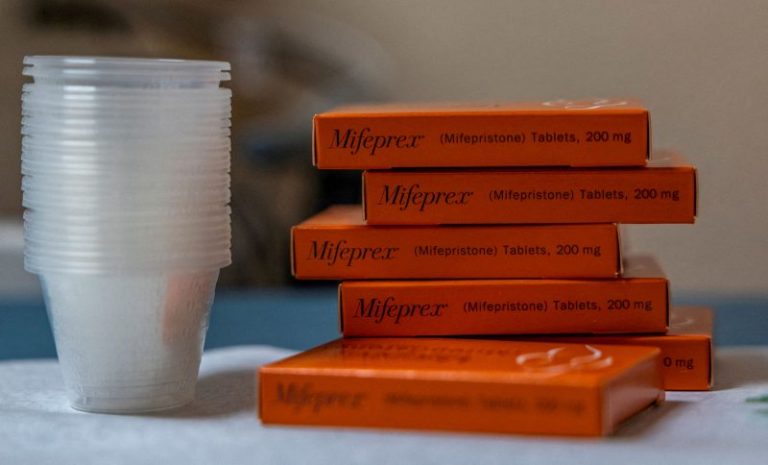
Twelve Democratic state attorneys general have sued the Food and Drug Administration in an attempt to loosen restrictions on the distribution of mifepristone, a pill used in medication abortions that has been at the center of the reproductive health debate after the Supreme Court overturned Roe v. Wade.
The lawsuit accuses the FDA of “singling out” and imposing “particularly burdensome” limitations on the distribution of mifepristone, which blocks the progesterone hormone that helps the body maintain a pregnancy. The legal challenge, led by the attorneys general for Washington and Oregon, was filed Thursday in a U.S. District Court in Washington state.
Mifepristone is the first part of a two-drug procedure that has become increasingly popular, as medication abortions (also known as medical abortions) now account for more than 50 percent of pregnancy terminations in the United States. Abortion rights supporters say the pills — which can be taken virtually anywhere and are transported easily — are key at a time when reproductive health care is increasingly hard for many Americans to access. Some physicians said they saw a surge in demand for abortion pills from a popular distributor after the overturn of Roe, which had established a fundamental right to abortion nationwide.
The drug, which is also used to manage miscarriages and treat Cushing’s syndrome, was first approved by the FDA in 2000. The agency said that mifepristone is safe and effective, with a 2016 medical review noting that “serious complications have proven to be extremely rare.” But mifepristone is also subject to restrictions known as the Risk Evaluation and Mitigation Strategy, a status it shares with just 60 other drugs, including fentanyl.
The program requires health care professionals and pharmacies to be certified before they can respectively prescribe and dispense such pills. Patients must also sign a form stating that they took the medication for the express purpose of ending a pregnancy.
The Washington suit seeks for a judge to declare that such categorization is “improper and discriminatory.” State Attorney General Bob Ferguson (D) said in a statement that the FDA’s rules expose “doctors, pharmacists and patients to unnecessary risk” and that the limits “have no basis in medical science.”
The FDA didn’t immediately return a request for comment early Saturday.
Arizona, Colorado, Connecticut, Delaware, Illinois, Michigan, Nevada, New Mexico, Rhode Island and Vermont are also involved in the legal action.
The Biden administration has sought to make these pills easier to access. The FDA this year moved to permit more pharmacies to dispense the pills, and the Justice Department said such drugs can legally be mailed to any state, including those where abortion is banned or sharply curtailed.
Mifepristone manufacturer GenBioPro has also sued West Virginia, where access to the drug and abortion is strictly limited. The company argues that the restrictions are in violation of federal law that permit the FDA to regulate drugs.
The efforts have met pushback by opponents of abortion. West Virginia Attorney General Patrick Morrisey (R) this week asked a judge to dismiss GenBioPro’s challenge, arguing that Congress has not given the FDA oversight over “this vast area of historically state regulation.”
Kansas Attorney General Kris Kobach (R), a hard-line social conservative, said this week that he had received a commitment from Walgreens pharmacy chain not to mail abortion pills into the state. A senior Walgreens official said in a letter released by Kobach that it “does not intend to dispense Mifepristone within your state and does not intend to ship Mifepristone into your state from any of our pharmacies.”
Antiabortion groups also filed a lawsuit against the FDA in November, in an attempt to force the agency to rescind its approval of mifepristone. They claim the FDA ignored side effects of the drug, and argue that pregnancy is not an “illness” and “abortion chemicals” do not provide a “therapeutic benefit.”
Legal experts have called the suit, filed in a U.S. court in Texas, baseless. It has come under the purview of District Court Judge Matthew Kacsmaryk, a Trump nominee known for his socially conservative views. If he rules in the plaintiffs’ favor, “64.5 million women of reproductive age” nationwide risk losing access to medication abortion, according to NARAL Pro-Choice America.
The plaintiffs in the Washington case, by contrast, are seeking that a judge declare mifepristone “safe and effective” and that the FDA was “lawful and valid” in approving the drug. The request raises the prospect of conflicting rulings from trial courts in different parts of the country on the pill’s safety.